What is clinical research?
Medical practice is based on preventive, diagnostic and therapeutic interventions, whose efficacy (benefits) and safety (absence of unwanted effects) must be proven through rigorous medical research.
While pre-clinical research is medical research carried out in a laboratory, without direct patient involvement and is aimed at collecting preliminary safety and efficacy data, clinical research involves people in so called “clinical studies” and is aimed at evaluating efficacy and safety of therapeutic interventions in the clinical setting.
Clinical studies
Some clinical studies are observational, meaning that researchers observe the “natural history” of patients treated according to routine clinical practice (standard of care), by collecting data on therapy outcomes and other patients’ and disease information. These studies are also called non-interventional or non-experimental.
Instead, other clinical studies are experimental (or interventional) and are called clinical trials.
When there is a state of genuine uncertainty regarding the best treatment for a specific health condition, clinical trials are needed. This condition is called “equipoise”. Clinical trials are the most reliable method for assessing the efficacy and safety of interventions, in order to establish which one to use in clinical practice. There are different types of clinical trials, according to the research question and to the applied methodology.
Some clinical trials test the new intervention without making comparisons. This depends on trial aims and phase. In this case the study is defined as uncontrolled or single-arm trial.
To avoid undue researcher influences in the assessment of the intervention’s effect, and/or the reporting of patients’ symptoms and outcomes, allocation to the intervention or control arms can be blinded or masked. If neither the doctor nor the patient know the allocation arm – the clinical trial is called “double blind” or “double masked”. On the opposite, in open-label trials everybody (researchers and participants) are aware of the allocation arm.
Types of clinical trials
Clinical trials allow to compare a new therapy (experimental) with another already used in clinical practice (control) to treat the health condition under study. These are called controlled clinical trials. In some situations, the experimental therapy is not new and has already been approved for other health conditions; in other cases, for which no standard is available, the control therapy could be a placebo, which is the use of an inactive substance. In controlled clinical trials patients are then allocated to the group receiving the new therapy (experimental group) or to the group receiving the control therapy (control group). Researchers often use the term “arm” to define these groups of patients. When allocation is performed at random (by chance, like flipping a coin) the studies are called randomized controlled trials. Random allocation is aimed at forming groups that are similar, so that the differences between the two groups on final outcomes are due to the different interventions received and not to pre-existing differences.
Some clinical trials test the new intervention without making comparisons. This depends on trial aims and phase. In this case the study is defined as uncontrolled or single-arm trial.
To avoid undue researcher influences in the assessment of the intervention’s effect, and/or the reporting of patients’ symptoms and outcomes, allocation to the intervention or control arms can be blinded or masked. If neither the doctor nor the patient know the allocation arm – the clinical trial is called “double blind” or “double masked”. On the opposite, in open-label trials everybody (researchers and participants) are aware of the allocation arm.
Study protocol. Ethics Committee and informed consent
When conducting a clinical study, researchers must always follow a written plan, also called “study protocol”. The protocol is defined before starting the study and provides a detailed description of the objectives, design, methodology, organization and conduction of the study.
Every clinical study has to be approved by an Ethics Committee before staring patient enrolment. The Ethics Committee is a multidisciplinary group of experts that evaluates the research protocol from the scientific and ethical points of view.
In order to decide participation in a clinical study, a patient must receive a document describing all the trial information from his/her doctor and, if the patient voluntarily decides to participate, she/he must sign and date the informed consent form. see Informed consent
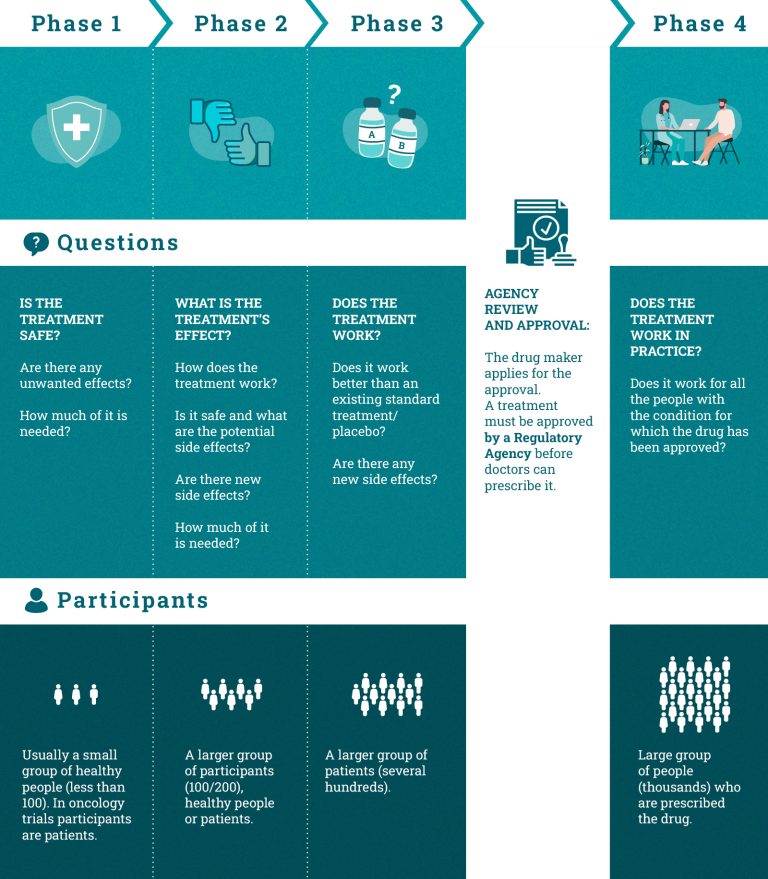
Phases of clinical trials
A new drug undergoes several testing phases during its clinical development process. If the drug proves to have an acceptable level of toxicity in relation to the expected benefit in one phase, then the subsequent trial phase can be initiated.
Each phase has a different focus:
Phase I trials test if a new therapy is harmless and answer the question: “Is it safe?” These studies are usually conducted on a limited number of healthy volunteers. However, certain Phase 1 trials (as happens in oncology) focus on patients rather than healthy people. The main objective is to assess potential treatments side effects and where and how they appear.
Phase II trials test the therapeutic activity of the treatment (i.e. its ability to produce the desired effects on the human body) and answer the question: “What is its effect?” They are conducted on a larger group of participants, healthy persons or patients.
Phase III trials test the efficacy of the new treatment and they answer the question “Does it work?” In this phase, the new treatment is compared with the standard one or with a placebo to test its efficacy and side effects, usually through a randomised controlled trial in selected patient groups.
Phase IV trials take place when the drug has been approved by the regulatory agencies and is available on the market. The purpose of this phase is to find more information about long-term benefits and side effects in widespread use in clinical practice. They answer the question “Does it work in clinical practice?”.
Basket trials
Basket trials are a new type of clinical trials focused on the treatment of cancer patients carrying a specific genetic mutation or molecular profile, for the purpose of evaluating a targeted therapy. Basket trials often use a methodological approach called “single-arm”, which is the evaluation of the efficacy of a drug without using a comparator.To know more about clinical studies, watch the short animated film designed and produced within the EU- funded ECRAN project: https://vimeo.com/69337236.
Clinical trials: important things to know
- “New treatments” are not necessarily better than standard ones in terms of efficacy and safety.
- Patients should be selected for a clinical trial if they are likely to benefit the most from the new treatment.
- For ethical reasons, placebo control groups are not allowed if a standard treatment already exists and patients can benefit from it. In such cases, the best standard treatment available must be used as a control.
- Clinical trials are ruled by international guidelines and legislation to protect participants and ensure their safety.
- Before conducting clinical trials involving patients, several studies (usually classified into “phases”) need to be carried out in the labs – within laboratory research and preclinical research. If these studies provide satisfactory findings, the drug is tested in clinical trials. The ultimate aim of a clinical trial is to move findings from bench to bedside, translating the research conducted in laboratories into concrete ways of helping patients.
How can I find the right clinical trial for me?
If you are considering taking part in a clinical trial, the first and easiest step is to ask your doctor, who is aware of your disease condition, knows all about your tests’ results, and will be able to refer you to the most suitable trials in local hospitals, research centres or universities.
Meeting other people who have been diagnosed with the same condition is also useful to gather information. Patients’ associations are usually very helpful and you can directly consult their websites.
You can also directly browse the clinical trial registers that collect clinical studies conducted around the world (https://www.clinicaltrials.gov/ ) or clinical trials conducted at the European level (https://www.clinicaltrialsregister.eu/ctr-search/search.). For each study, you can read a summary of the study protocol and find out study locations and specific contact information. If you find a clinical trial in which you might be interested in, the best thing to do is to talk with your doctor.
In any case, always go through the whole informed consent process before taking your final decision. Even though this might be a time-consuming process, it is essential to make sure you fully understand what you are signing up for.
Source: ECRAN – European Communication on Research Awareness Needs – project funded by European Union’s Seventh Framework Programme.